Todays Article: https://www.fiercebiotech.com/biotech/gilead-and-rivals-deep-phase-3-fda-unveils-draft-guidance-nash-drug-development
So I was first a bit hazy as to what NASH Drugs are: NASH (Non-Alcoholic Steatohepatitis). Essentially the disease is as such: lipids accumulate in vesicles displacing the cytoplasm, displacing the nucleus and severe versions cause the cells to burst. A person’s inability to process lipids is what can cause this. This can stem from diabetes, protein malnutrition or obesity. As we commonly think of the liver when it comes to processing fat, this is where we can see the effect the most heavily generally:
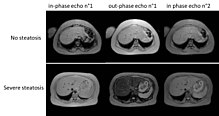
The progression of the disease starts with steatosis. It is generally isolated to an organ. It then can progress to over decades into steatohepatitis and then eventually lead to cirrhosis (scarring and destruction of liver tissue). As of writing this, there are currently no treatments for NASH. There is a high prevalence of NASH, and high morbidity from this disease. It is difficult to get liver transplants as well so this medicine could make great strides for those who suffer from NASH.
So what did the FDA say anyway? Full Text
They made new regulations for what they expect from the Phase II and Phase III Trials. Some companies are currently in Phase II and Some companies are in Phase III. So what are the expectations?
Recommendation:
Attach a biomarker to the drug. People don’t like biopsies as they are invasive and can often lead to morbidity. It’d be nice to have an easy way to track the drug through some kind of biomarkers and accelerate drug development into NAFLD.
Phase II:
Pick a patient population that will be represented such as those who have Diabetes. Testing out more dosages may allow them to combine phase 2 and 3. The clinical trials should last 12-18 months. Anything shorter should be explained why it was so short.
Phase III:
If the product can be fast-tracked if it can reduce the NASH level or and not worsening on fibrosis and an improvement of one level of liver fibrosis.
Key Players in Phase III Trials
Allergan, Genfit, Gilead, Intercept Pharmaceuticals:
These 4 comapnies have already satisfied many of the FDA requests for acceleration
Key Players in Phase IIb Trials (Testing for dosage)
BMS, Inventiva Pharmaceuticals.
There companies look to fulfill what the FDA asks of them in Phase IIb studies as the FDA has shown skepticism for the time periods that the previous companies had spent on their clinical trials. The FDA expects a longer trial period.\
Novartis
Paid 50 million for Conatus and it failed Phase 2b
My thoughts and lingering questions (hopefully I’ll learn more in time to come)
This seems like a great untapped field for the companies to tap into and it seems to fit in nicely with many drug portfolios. This product is an FXR agonist so I assume that it may be some kind of antibody? Considering there is no current treatment for NASH, it makes me wonder what the costs will be and who will come out ahead. Even the late players like BMS can still have an advantage and it truly speaks to the marketing teams that will be on the job to sell the different value propositions to physicians. This looks great as a product that can help turn this disease into more of a “chronic condition” if not eliminate it all together? I’m unsure about the endpoints for the patients. Do the patients have to keep taking the products? With so many players entering the market, I wonder which ones will have the best efficacy, which ones will have dominance in the market, how physicians will choose between the molecules. The article does mention that Allergan’s is slightly different than Gilead’s but I’m still unsure of how.
As this was my first piece, it actually took me a while to read through everything, understand it vaguely and write about it. I hope future pieces will show a bit more thought and depth as i learn more about the industry.